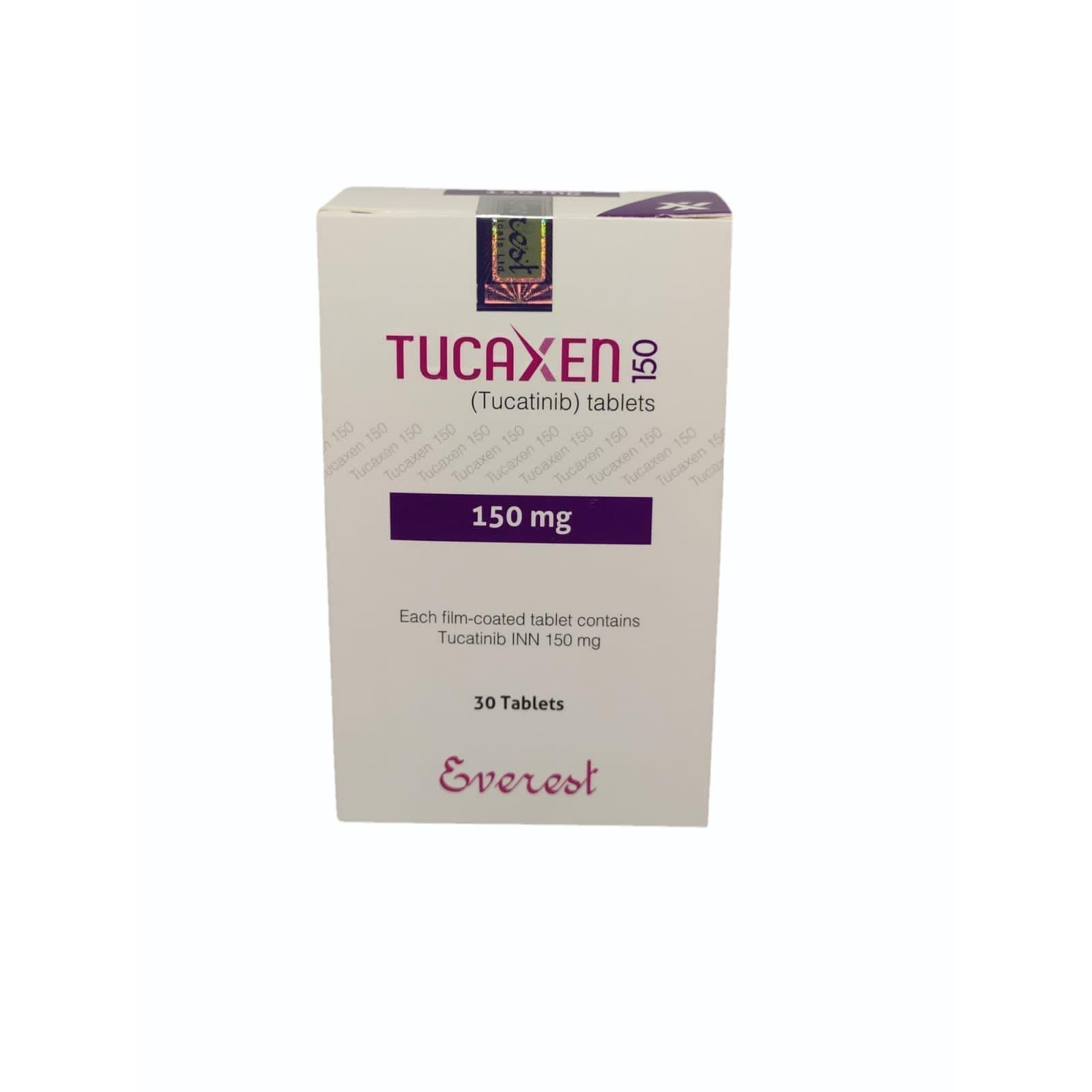
Tucatinib 150mg We Ashtavinayaka Export Is The Leading Supplier, Dealer, Exporter For Osimertinib 80 Mg Tablets In London , New York, Johannesburg, Switzerland , TEXAS, CALIFORNIA, LOSS ANGELES, CHIICAGO, NEW YORK, WASHINGTON, UNITED KINGDOM, SOUTH AFRICA, LAS VEGAS.. Tucatinib is used with trastuzumab (Herceptin) and capecitabine (Xeloda) to treat a certain type of hormone receptor–positive breast cancer that has spread to other parts of the body and cannot be treated with surgery in adults who have already been treated with at least one other chemotherapy medication TEXAS, CALIFORNIA, LOSS ANGELES, CHIICAGO, NEW YORK, WASHINGTON, UNITED KINGDOM, SOUTH AFRICA, LAS VEGAS, Dubai, Abu Dhabi, Sharjah, Umm Al-Quwain, Fujairah, Ajman And Ra\\\\\'s Al-Khaimah, Saudi Arabia, TEXAS, CALIFORNIA, LOSS ANGELES, CHIICAGO, NEW YORK, WASHINGTON, UNITED KINGDOM, SOUTH AFRICA, LAS VEGAS.. it is used in breast cancer called human epidermal growth factor receptor-2 (HER2) positive breast cancer. TUKYSA is used with the medicines trastuzumab and capecitabine, when your cancer has spread to other parts of the body such as the brain (metastatic), or cannot be removed by surgery, and you have received one or more anti-HER2 breast cancer treatments. a type of colorectal cancer called RAS wild-type HER2 positive colorectal cancer. TUKYSA is used with the medicine trastuzumab, when your cancer has spread to other parts of the body (metastatic), or cannot be removed by surgery, and you have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working. Strength 150 mg Brand Name tucaxen 150mg Composition tucaitinib Form Tablets Packaging Type Bottle Prescription/Non prescription Prescription This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.

This is your website preview.
Currently it only shows your basic business info. Start adding relevant business details such as description, images and products or services to gain your customers attention by using Boost 360 android app / iOS App / web portal.

Submit Your Enquiry